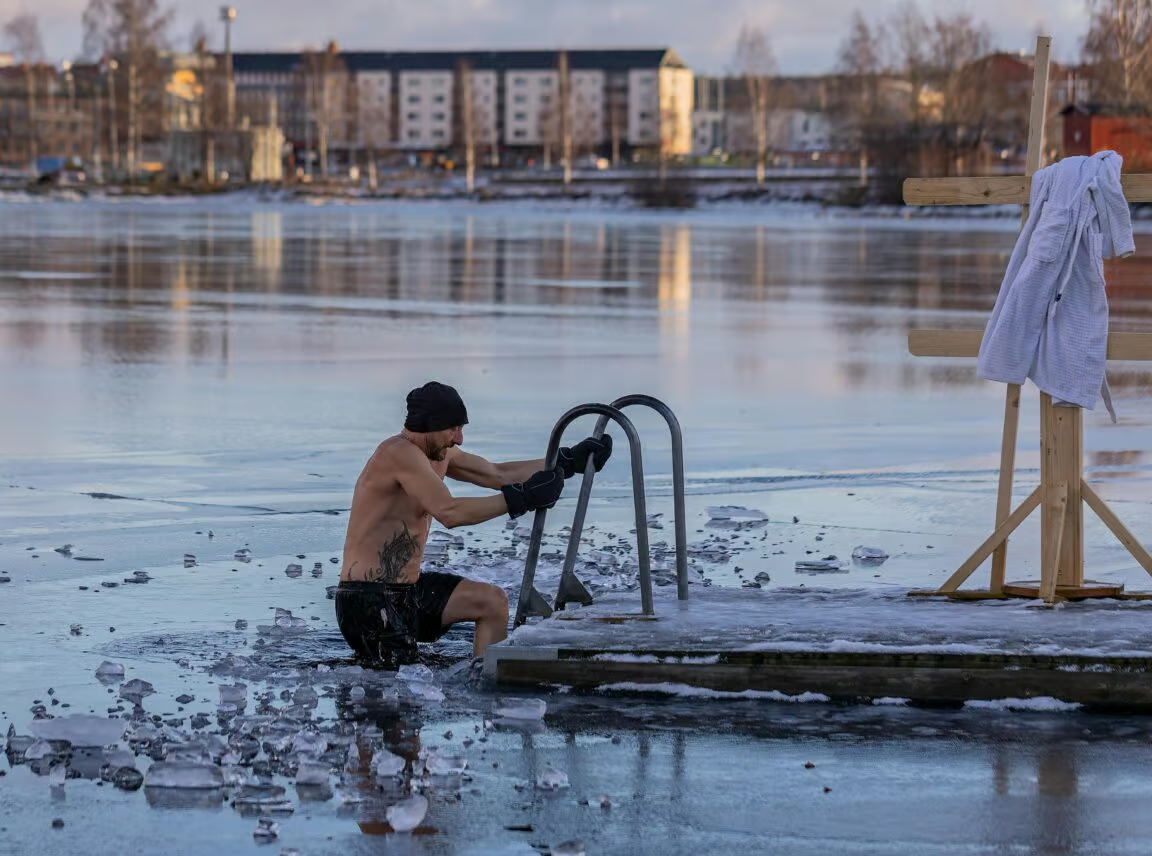
Why Cold and Heat Shock Proteins Matter
Heat and cold shock proteins aren’t just scientific curiosities. They are stress-response molecules that can transform how your body recovers, regulates inflammation, and adapts to training. Whether triggered by saunas, cold plunges, intense workouts, or fasting, these proteins help your cells stay resilient under pressure.
For those pursuing sustainable fat loss and improved metabolic health, understanding these cellular protectors can offer new tools for boosting results without overtraining or extreme dieting.
Here is how cold shock proteins (CSPs) and heat shock proteins (HSPs) actually work and why they matter for recovery, body composition, and long-term vitality.
What Are Heat and Cold Shock Proteins?
Shock proteins are molecular chaperones. These are proteins your cells produce in response to stress. Their job is to stabilize other proteins, repair damaged structures, and maintain cell function when conditions get extreme (1).
- Heat shock proteins (HSPs) are triggered by high temperatures, exercise, oxidative stress, and inflammation.
- Cold shock proteins (CSPs) are triggered by cold exposure, including ice baths, cryotherapy, and environmental cold.
Despite the name, these proteins are not only activated by temperature changes. They also respond to metabolic stress, including fasting, calorie restriction, and intense physical exertion (2).
Heat Shock Proteins and Fat Loss
When you’re exposed to heat (such as in a sauna or during intense workouts), HSPs increase in number and activity.
This has several benefits:
- Reduced oxidative stress: HSPs help clear damaged proteins and support mitochondrial health (3).
- Improved insulin sensitivity: Some forms of HSPs help reduce insulin resistance by dampening inflammatory pathways like TNF-α and NF-κB (4).
- Enhanced muscle preservation: HSP70, in particular, helps protect muscle cells from breakdown during exercise or metabolic stress (5).
Key takeaway: Saunas and heat exposure help maintain muscle mass, reduce inflammation, and promote recovery. These are essential during fat loss phases when overtraining and oxidative stress can stall progress.
Cold Shock Proteins and Resilience
Cold shock proteins like RNA-binding motif protein 3 (RBM3) and CIRP (cold-inducible RNA-binding protein) help your cells adapt to cold by:
- Stabilizing RNA and DNA expression
- Improving synaptic plasticity and brain health
- Reducing inflammation in tissues under physical stress (6)
Cold exposure also activates norepinephrine, a hormone that increases alertness, boosts mood, and stimulates brown fat thermogenesis — the body’s process of burning fat to generate heat (7).
Key takeaway: Cold exposure isn’t just about willpower. It may support fat oxidation, recovery, and cognitive resilience through specific cold shock proteins and hormonal pathways.
Hormesis: Why Stress Isn’t Always Bad
Both HSPs and CSPs operate under the principle of hormesis, where small doses of controlled stress lead to a stronger, more adaptive response. This is the same concept behind resistance training, fasting, or even Zone 2 walking.
Mild stress, followed by adequate recovery, tells your cells to:
- Upgrade antioxidant defenses
- Repair damaged proteins
- Boost mitochondrial efficiency
- Reduce low-grade inflammation
These adaptations are exactly what you want if you are aiming for long-term body composition change, not just short-term weight loss.
Want a clear, effective path to sustainable fat loss?
Sign up for the PlateauBreaker™ Plan and start your fat-loss journey today.
Neuroprotective and Anti-Aging Benefits
Both heat and cold shock proteins appear to play roles in slowing aging and protecting brain function.
In studies:
- HSP70 has been shown to reduce tau aggregation in Alzheimer’s models (8).
- RBM3, a cold shock protein, helps preserve synapses in neurodegenerative disease and is associated with longer lifespan in animal models (6).
These proteins may also increase brain-derived neurotrophic factor (BDNF), a compound that supports neuroplasticity, learning, and emotional resilience.
Protein Folding: The Front Line of Cellular Repair
One of the most important roles of shock proteins, and one often overlooked, is protecting how your proteins fold under stress.
Proteins only function if they’re folded correctly. Heat, cold, oxidative stress, and even intense training can cause them to misfold or clump. These misfolded proteins are not just broken. They’re toxic. They impair metabolism, trigger inflammation, and are linked to neurodegenerative diseases like Alzheimer’s (9).
Heat and cold shock proteins act as molecular guardians:
- HSPs help fold new proteins correctly, refold damaged ones, and shuttle beyond-repair proteins to cellular cleanup systems
- CSPs stabilize RNA during cold stress, preserving the instructions your cells need to make proteins under pressure
This protein quality control system is essential for:
- Muscle recovery
- Mitochondrial performance
- Brain function
- Slowing the cellular aging process
💡 Key takeaway: Shock proteins help your body handle stress, not by masking it, but by repairing its damage at the molecular level (10).
How to Activate Shock Proteins Safely
You don’t need to suffer through extremes to reap the benefits. Start with moderate, consistent exposure and listen to your recovery cues.
Simple strategies:
- Sauna sessions: 15–20 minutes, 2–4 times per week
- Cold showers or plunges: 30 seconds to 3 minutes, several times per week
- Exercise: Especially strength training and high-intensity intervals, which stimulate both heat and metabolic stress responses
- Fasting: 14–18 hour windows can upregulate protective stress proteins without harming muscle (when done properly)
What to Avoid:
- Overuse of cold post-exercise (may blunt muscle-building signaling if overused)
- Pairing multiple stressors (cold + fast + HIIT) without recovery
- Ignoring signs of overtraining or burnout
✏︎ The Bottom Line
Shock proteins are not hacks. They are how your body builds resilience. From better fat burning and insulin sensitivity to cognitive protection and recovery, HSPs and CSPs support every major system involved in sustainable fat loss.
At PlateauBreaker, we teach fat loss as a system, not a calorie deficit. Incorporating strategic heat and cold exposure may be the missing link that helps your metabolism stay flexible, your mind stay sharp, and your body keep progressing.
Download our free eBook
10 Weight Loss Myths That Are Keeping You Stuck – And How to Break Free
Train hard. Recover smarter. Build the biology that burns fat.
Bibliography
(1) Jolly, C, and R I Morimoto. “Role of the heat shock response and molecular chaperones in oncogenesis and cell death.” Journal of the National Cancer Institute vol. 92,19 (2000): 1564-72. doi:10.1093/jnci/92.19.1564. https://pubmed.ncbi.nlm.nih.gov/11018092/
(2) Liu, Jiahao et al. “Cold-Induced RNA-Binding Protein and RNA-Binding Motif Protein 3: Two RNA Molecular Chaperones Closely Related to Reproductive Development and Reproductive System Diseases.” Protein and peptide letters vol. 30,1 (2023): 2-12. doi:10.2174/0929866530666221124122507. https://pubmed.ncbi.nlm.nih.gov/36424802/
(3) Salo, D C et al. “HSP70 and other possible heat shock or oxidative stress proteins are induced in skeletal muscle, heart, and liver during exercise.” Free radical biology & medicine vol. 11,3 (1991): 239-46. doi:10.1016/0891-5849(91)90119-n. https://pubmed.ncbi.nlm.nih.gov/1937141/
(4) Henstridge, Darren C et al. “Chaperoning to the metabolic party: The emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes.” Molecular metabolism vol. 3,8 781-93. 30 Aug. 2014, doi:10.1016/j.molmet.2014.08.003. https://pubmed.ncbi.nlm.nih.gov/16309849/
(5) Broome, Caroline S et al. “Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation after nondamaging contractile activity.” FASEB journal : official publication of the Federation of American Societies for Experimental Biology vol. 20,9 (2006): 1549-51. doi:10.1096/fj.05-4935fje. https://pubmed.ncbi.nlm.nih.gov/16723383/
(6) Peretti, Diego et al. “RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration.” Nature vol. 518,7538 (2015): 236-9. doi:10.1038/nature14142. https://pubmed.ncbi.nlm.nih.gov/25607368/
(7) Cypess, Aaron M et al. “Identification and importance of brown adipose tissue in adult humans.” The New England journal of medicine vol. 360,15 (2009): 1509-17. doi:10.1056/NEJMoa0810780. https://pubmed.ncbi.nlm.nih.gov/19357406/
(8) Patterson, Kristina R et al. “Heat shock protein 70 prevents both tau aggregation and the inhibitory effects of preexisting tau aggregates on fast axonal transport.” Biochemistry vol. 50,47 (2011): 10300-10. doi:10.1021/bi2009147. https://pubmed.ncbi.nlm.nih.gov/22039833/
(9) Abramov, Andrey Y et al. “Interaction of Oxidative Stress and Misfolded Proteins in the Mechanism of Neurodegeneration.” Life (Basel, Switzerland) vol. 10,7 101. 30 Jun. 2020, doi:10.3390/life10070101. https://pmc.ncbi.nlm.nih.gov/articles/PMC7400128/
(10) Powers, Evan T et al. “Biological and chemical approaches to diseases of proteostasis deficiency.” Annual review of biochemistry vol. 78 (2009): 959-91. doi:10.1146/annurev.biochem.052308.114844. https://pubmed.ncbi.nlm.nih.gov/19298183/




